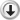Ergosterol
Page 1 of 1
 Ergosterol
Ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol), a sterol, is a biological precursor (a provitamin) to vitamin D2. It is turned into viosterol by ultraviolet light, and is then converted into ergocalciferol, which is a form of vitamin D.[1]
Ergosterol is a component of fungal cell membranes, serving the same function that cholesterol serves in animal cells. Because ergosterol is present in fungal cell membranes yet absent in animal cell membranes, it is a useful target for antifungal drugs. Ergosterol is also present in the cell membranes of some protists, such as trypanosomes.[2] This is the basis for the use of some antifungals against West African sleeping sickness.
Amphotericin B is an antifungal drug that targets ergosterol. It binds physically to ergosterol within the membrane, and, thus, creates a polar pore in fungal membranes. This causes ions (predominantly potassium and protons) and other molecules to leak out, which will kill the cell.[3] Amphotericin B has been replaced by safer agents in most circumstances but is still used, despite its side-effects, for life-threatening fungal or protozoan infections.
Miconazole, Itraconazole, and Clotrimazole work in a different way, inhibiting synthesis of ergosterol from lanosterol. Ergosterol is a smaller molecule than lanosterol; it is synthesized by combining two molecules of farnesyl pyrophosphate, a 15-carbon-long terpenoid, into lanosterol, which has 30 carbons. Then, two methyl groups are removed, making ergosterol. The "azole" class of anti-fungal agents inhibit the enzyme that performs these demethylation steps in the biosynthetic pathway between lanosterol and ergosterol.
brochure printing
franchises
Ergosterol is a component of fungal cell membranes, serving the same function that cholesterol serves in animal cells. Because ergosterol is present in fungal cell membranes yet absent in animal cell membranes, it is a useful target for antifungal drugs. Ergosterol is also present in the cell membranes of some protists, such as trypanosomes.[2] This is the basis for the use of some antifungals against West African sleeping sickness.
Amphotericin B is an antifungal drug that targets ergosterol. It binds physically to ergosterol within the membrane, and, thus, creates a polar pore in fungal membranes. This causes ions (predominantly potassium and protons) and other molecules to leak out, which will kill the cell.[3] Amphotericin B has been replaced by safer agents in most circumstances but is still used, despite its side-effects, for life-threatening fungal or protozoan infections.
Miconazole, Itraconazole, and Clotrimazole work in a different way, inhibiting synthesis of ergosterol from lanosterol. Ergosterol is a smaller molecule than lanosterol; it is synthesized by combining two molecules of farnesyl pyrophosphate, a 15-carbon-long terpenoid, into lanosterol, which has 30 carbons. Then, two methyl groups are removed, making ergosterol. The "azole" class of anti-fungal agents inhibit the enzyme that performs these demethylation steps in the biosynthetic pathway between lanosterol and ergosterol.
brochure printing
franchises
tungduong_9102- Posts : 230
Join date : 2010-10-15
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum